Gold is known for its beauty and rarity, but beyond jewelry and luxury, it also plays a crucial role in science and technology. One of the most important questions often asked is whether gold can conduct electricity. The answer is yes—gold is an excellent conductor. However, its ability to conduct electricity is not the only reason it is used in electronics. Understanding why gold conducts electricity and how it compares with other metals reveals much about its unique role in modern life.
The Science of Conductivity
Electrical conductivity is the ability of a material to allow the flow of electric current. Metals such as copper, silver, and gold are all good conductors because of their atomic structure. In metals, electrons are not tightly bound to atoms. Instead, they form a “sea of electrons” that can move freely when voltage is applied. Gold, like silver and copper, belongs to this group. It has high electrical conductivity, which means electrons flow easily through it, making it useful for connections that require reliability.
Gold Compared to Other Metals
While silver is technically the best conductor of electricity, and copper comes second, gold is not far behind. What makes gold special is its resistance to corrosion. Silver tarnishes when exposed to air and sulfur, and copper oxidizes to form a green surface layer. Gold, on the other hand, does not rust, tarnish, or oxidize easily. This chemical stability makes gold more valuable for use in electronics, especially in environments where durability is essential. In fact, gold-coated connectors are common in computers, smartphones, and even spacecraft.

Applications of Gold in Technology
Gold’s ability to conduct electricity reliably has made it essential in advanced technologies. In telecommunications, thin layers of gold are used to coat connectors and circuit boards. In aerospace engineering, gold is used to ensure stable performance under extreme conditions. Even in medicine, tiny amounts of gold are used in specialized electronic devices, such as pacemakers, where reliability is a matter of life and death. These applications demonstrate that gold’s conductivity, combined with its resistance to damage, makes it irreplaceable in certain fields.
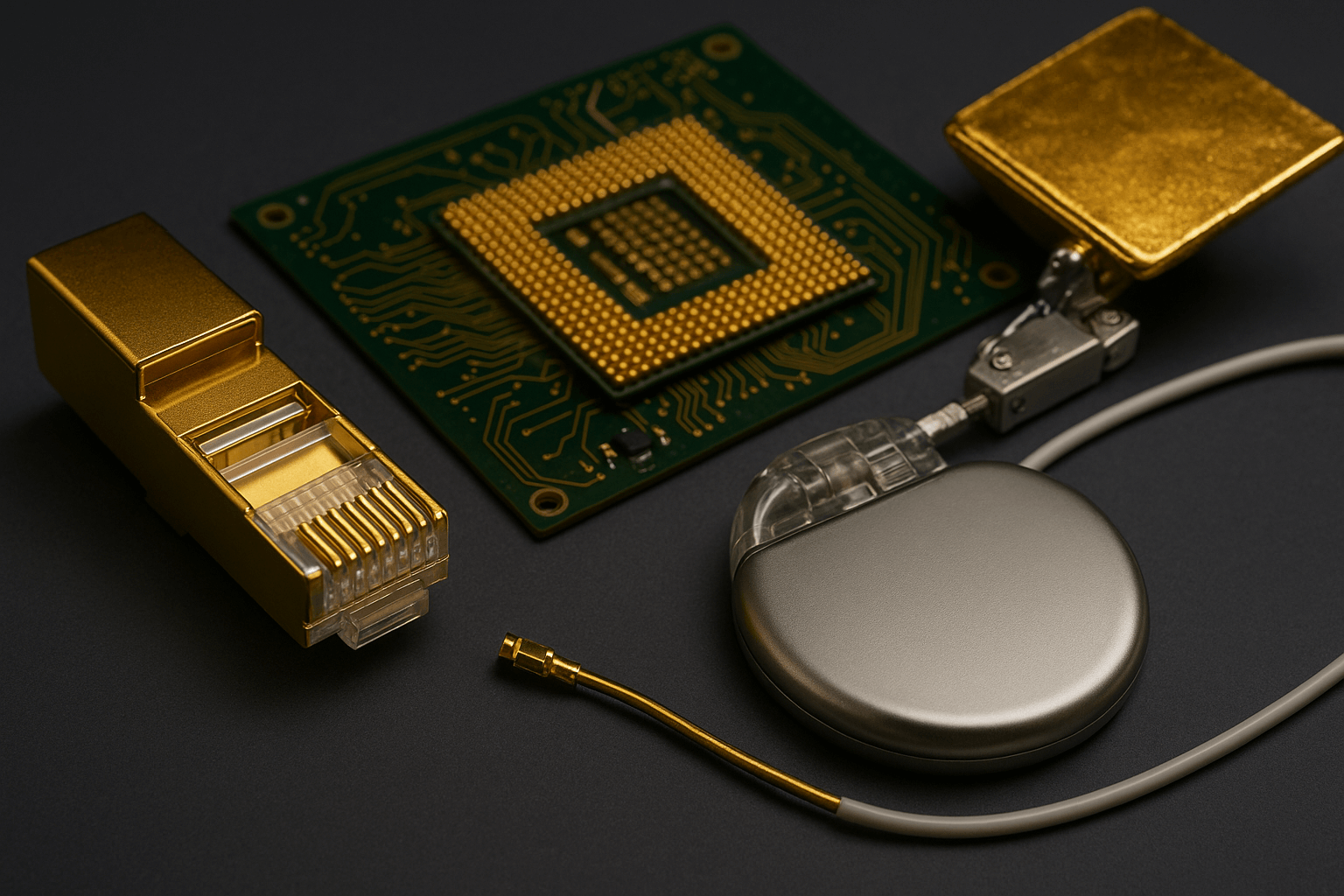
Why Gold Is Chosen Over Cheaper Metals
Copper and aluminum are widely used because they are less expensive, but they cannot match gold’s long-term stability. For example, copper wires may lose efficiency over time due to oxidation, while gold connections remain clean and efficient even after years of use. This durability explains why gold, despite its higher cost, continues to be used in critical systems where failure is not an option

Gold does conduct electricity, and while it is not the absolute best conductor, it is among the most reliable. Its unique combination of conductivity, resistance to corrosion, and chemical stability makes it indispensable for modern technologies. From the circuits in a smartphone to the connectors in spacecraft, gold proves that its value is more than ornamental. It is not just a symbol of wealth but also a foundation of technological progress. online gold trading in dubai
FAQ
1. Is gold a better conductor than copper or silver?
No. Silver is the best conductor, followed by copper, but gold’s corrosion resistance makes it more reliable in practice.
2. Why is gold used in electronics if it is expensive?
Gold does not tarnish or corrode, ensuring reliable long-term conductivity, especially in sensitive systems.
3. Where is gold used in technology?
Gold is used in circuit boards, connectors, medical devices, and aerospace equipment due to its stability and conductivity.
4. Can pure gold wires conduct electricity?
Yes, pure gold wires conduct electricity efficiently, though they are usually thin or used as coatings due to cost.
